BMF announces study using postbiotics in AMD patients
- Javier Asarta, CEO of the Igen Biolab Group: “AMD affects some 700,000 people in Spain and currently has no treatment”.
- Dr Jordi Monés, study author: “As well as the medical benefits for patients, it reduces the socio-economic burden by preventing or improving the advanced stages of AMD”.
- If the study with product from the Igen Biolab Group achieves good results, a multicentre trial will be conducted.
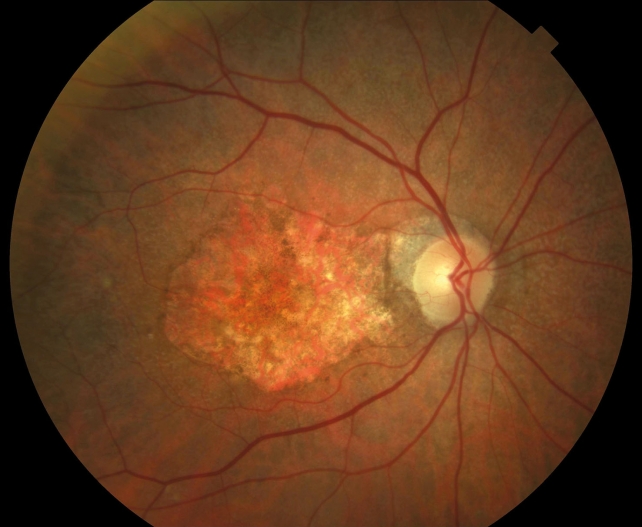
The pilot study assesses the progression of age-related macular degeneration (AMD) and the response to supplementation with postbiotics in patients with the pathology. It is led by Dr Jordi Monés, specialist and Director of the Institut de la Màcula and the Barcelona Macula Foundation: Research for Vision in conjunction with the Igen Biolab Group biotechnology laboratory. Taking place at the Institut de la Màcula, located on the campus of the Teknon Medical Centre, the study will lead to the conducting of a multicentre trial if results prove encouraging.
The rationale behind this clinical study is based on the following premises: “Genetic predisposition only partially responds to the incidence, and especially, to the progression and severity of AMD. We know that epigenetic factors are crucial in determining the progression and severity of the disease. One of the best ways of modulating these factors is to focus on the microbiome. A good approach is by using postbiotics, a functional food that could modulate the epigenome of patients with AMD, macular atrophy and choroidal neovascularisation without the risks associated with modifying the microbiome through the use of live bacteria and, therefore, could change the course of the disease”, Dr Monés explains.
AMD, the leading cause of blindness in the over-50s
AMD is the leading cause of total blindness in people of over 50 in developed countries. “Currently, there are no treatments for a disease that causes progressive loss of vision so if the results are favourable, it would be a major step towards alleviating a disease that affects some 700,000 people in Spain (1.5% of today’s population) and is one of the blindness-associated pathologies that will grow most in coming years1”, says the CEO of Igen Biolab Group, Javier Asarta.
“In addition to the personal medical benefits for patients, the functional improvement and the consequent reduction of the socio-economic burden by preventing or improving the advanced stages of AMD -either for patients, their families, or society as a whole- would be made enormous by the use of a therapy with minimal side effects that is administered easily and safely”, Dr Monés adds.
AMD is a true epidemic on the rise, one that currently affects about 20% of those who are 85 and over. At present, AMD affects 8.7% of the world population, estimated to be 196 million in 2020 and increasing to 288 million in 2040. Dr Monés explains that “AMD is a complex multifactorial disease with genetic, epigenetic and environmental components, as well as specific cellular senescence processes”.
The postbiotic approach, an alternative
To overcome the risks, limitations and reliance on the use of live intestinal bacterial cells, another approach has emerged recently. “The same beneficial anti-inflammatory, immunomodulatory, antiproliferative and antioxidant effects, while avoiding the risks of live bacteria, can be obtained through the use of cell lysates, which are also known as postbiotics,” Dr Monés says.
“Postbiotics have been shown to mimic the effects of probiotics on health, while avoiding the need to administer live microorganisms, which can carry significant risks such as a local inflammatory response similar to that of salmonella, bacteraemia and fungaemia, and the possible transfer of the antibiotic resistance gene. Therefore, the postbiotic approach represents a safer alternative with less risk,” he adds.
About the Igen Biolab Group: https://www.igenbiolabgroup.com/