At the 15th ISOPT Clinical, Dr Monés describes the origin and evolution of AMD and the factors involved in it
The Director, principal investigator and one of the founding trustees of the Barcelona Macula Foundation, Dr Jordi Monés MD, PhD, was a protagonist at the 15th ISOPT Clinical. At the event, he embarked on a comprehensive review of the pathogenesis of AMD and the new therapies for treating retinal diseases, such as gene therapy
Dr Jordi Monés MD, PhD, the Director, principal investigator and one of the founding trustees of the Barcelona Macula Foundation (BMF), was a protagonist at the prestigious 15th International Symposium on Ocular Pharmacology and Therapeutics Clinical: The Retina Chapter (ISOPT Clinical), a meeting point for analysing the pharmacological therapies and treatments of this specialty held in Valencia on 7-9 November. Dr Monés gave three lectures, the first of which was entitled “Simultaneous neutralization of angiopoietin-2 (Ang-2) and vascular endothelial growth factor-A (VEGF-A) with faricimab: a review of the phase 2 clinical trials in neovascular age-related macular degeneration (nAMD) and diabetic macular edema (DME)”, where he reviewed the recent very positive results of Faricimab, the first bispecific antibody that targets at the same time two different cytokines: the angiopoietin-2 and the VEGF-A. With this dual approach this drug has demonstrated much longer durability than current available anti_VEGF drugs for nAMD, and higher efficacy in addition to durability for DME.
The other presentations were “Review the pathogenesis of AMD and the pathways to CNV and GA” and “Novel gene therapy approaches for inherited retinal diseases” in which he analysed the hypothesis of drusen ooze and gene therapy for retinal diseases.
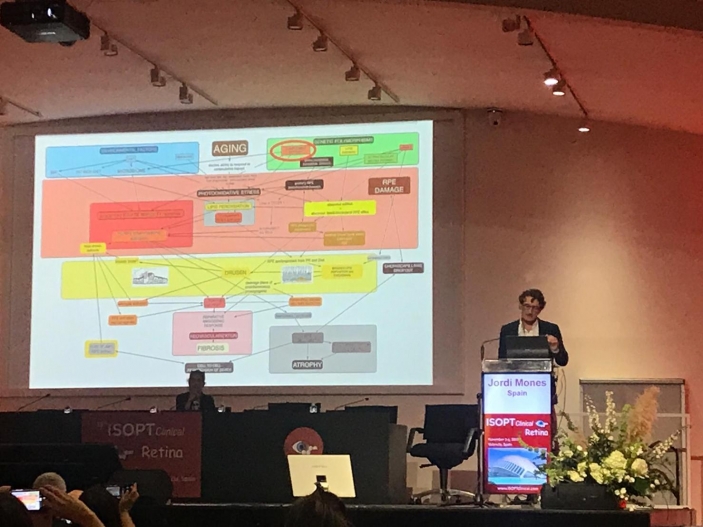
“Review the pathogenesis of AMD and the pathways to CNV and GA” formed part of a round table on age-related macular degeneration (AMD). Here, Dr Monés considered the highly complex pathogenesis of this pathology and provided an exhaustive description of the disease’s origin and evolution, together with all the factors involved in it and the multiple interlinked pathways to CNV and GA, pointing the most crucial spots where to target future therapies.
In this regard, Dr Monés explained that the retina is one of the most active body tissues from a metabolic point of view and oxidative stress. This together with the genetic predisposition on complement overactivation, on abnormal lipid efflux metabolism and on primary mitochondrial damage leads to toxic end products of lipid peroxidation that trigger the inflammatory cascade that eventually causes cell death.
“Besides targeting inflammation by anti Complement therapies, other key players to address are lipid peroxidation, abnormal lipid cholesterol RPE eflux and mitochondrial oxidative stress. Also, no matter which the primary insult is, targeting drusen and Bruch’s membrane lipid deposition would stop the vicious toxic cycle of inflammasome activation and RPE death by drusen leaking extracts through RPE defects ( drusen ooze),” Dr Jordi Monés MD, PhD explained.
In “Novel gene therapy approaches for inherited retinal diseases“, Dr Monés presented the novel MINI GENE strategy in Gene Therapy by IVERIC bio, to overcome the size packaging capacity limitation of less than 5 kb of the adeno-associated virus vectors. The Medical Director of Barcelona Macula Foundation explained that it is “a promising approach for inherited retinal diseases with large gene such as the Stargardt’s disease, that has not been able yet to be addressed with gene therapy due to this critical limitation.” “Very promising expectations to try to prevent disease progression and visual loss in these young affected population”, Dr Jordi Mones MD, PhD added. The clinical trials in humans are likely to start in less than 5 years, after validation of safety and efficacy in experimental models.
Other gene therapy approaches discussed were the potential best-in-class strategy of knowdown and replacement with the same single AAV vector that it is in pre-clinical stage for rhodospsin mediated autosomal dominant retinitis pigmentosa. With this strategy suppression of endogenous mutant toxic rhodopsin protein and replacement with healthy rhodopsin protein is done at the same time with the same AAV vector. Furthermore, this approach is mutation independent. Clinical trials expected to start in 2020.
Other gene therapy that will start in 2021 will address BEST-1 related retinal diseases. Resolving the disease in naturally occurring Best related retinal degeneration in dogs have been the proof-of-concept that reversal of the disease is possible.
In addition to Dr Mones’ three lectures, the Barcelona Macula Foundation was represented by its ophthalmologist and researcher, Dr Lucía Lee Ferraro MD, who also spoke at the 15th ISOPT Clinical with a presentation entitled “Transplantation of human iPS cells-derived RPE cell suspension into the subretinal space of a swine model of geographic atrophy”. This is linked to the ADVANCE(CAT) project of which the Barcelona Macula Foundation forms part, together with the Universitat de Barcelona (UB), the Centre OF Regenerative Medicine in Barcelona (CMRB), the Leitat Foundation, the Banc de Sang i Teixits (BST), Ferrer International, Bioiberica, the August Pi i Sunyer Biomedical Research Institute (IDIBAPS), the Bosch i Gimpera Foundation (FBiG) and the Health Sciences Research Institute of the Germans Trias i Pujol Foundation (IGTP).