What is metabolomics in the context of AMD?
Age-related macular degeneration (AMD) is a leading cause of blindness in developed countries, and early diagnosis may lead to interventions that slow or decrease the risk of progression towards the late stages of the disease, where severe visual loss may ensue. Researchers of the European Union-funded Eye-Risk study, in which the Barcelona Macula Foundation participates, are looking at clues in the metabolism that lead to an early disease detection.
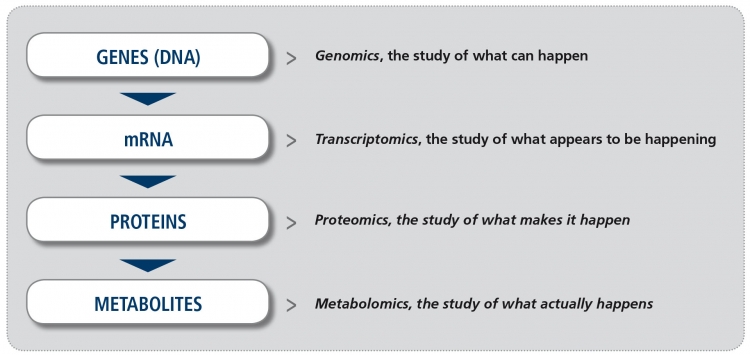
In recent years, increased availability of new technology platforms to study biological samples and greater potential to analyze the enormous amount of information derived from them (the so-called “big data”), have made the study of “omics” a reality. The suffix “omics“ means the study of all processes involved in a given field of study in Biology. For example, metabolomics is the study of all chemical processes involving metabolites, the small products of metabolism. The four major fields in “omics” (Figure) are genomics (the study of all DNA), transcriptomics (the study of all mRNA), proteomics (the study of all proteins) and metabolomics. Other omics fields involve the microbiome and the lipidome, for example.
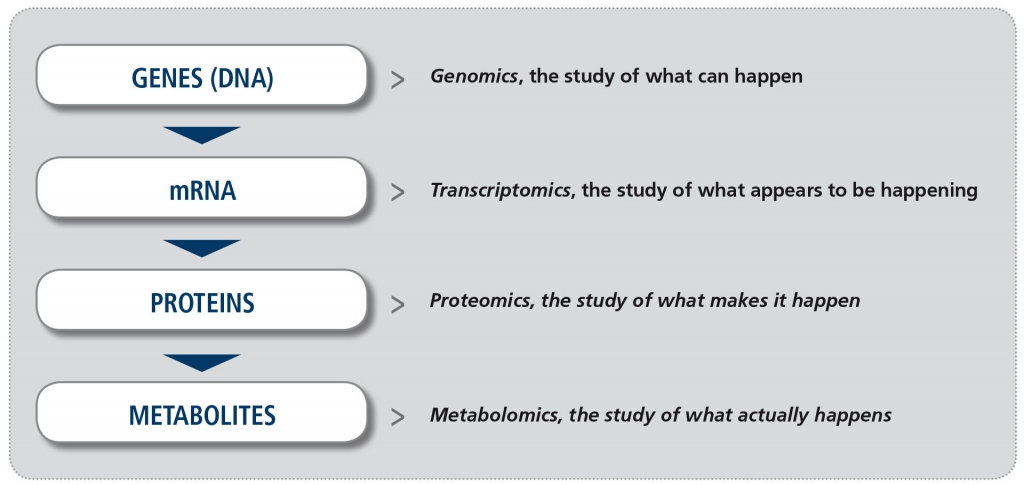
Figure. The major “omics” fields (adapted from Balashova EE et al. J Pers Med 2018).
Metabolomics is the least developed of all these fields. However, unlike other “omics” approaches it reflects the current biological activity of the cells, and therefore it is regarded as being most closely related to the patient phenotype (what is actually seen in the patient’s retina). Coupled with the recent feasibility of simultaneously evaluating hundreds to thousands of metabolites, the field of metabolomics has experienced a renewed interest in the past years.
The samples to be studied in metabolomics can be drawn from the peripheral blood, the urine or from the eye (tears, vitreous or aqueous humor), but generally speaking the closer to the tissue of interest, the better. The samples are usually analyzed by several techniques that use a mass spectrometer (a device that measures masses within a sample to characterize its composition), although other approaches can also be used. The development of new non-invasive imaging methods (i.e., Fluorescence lifetime imaging ophthalmoscopy, FLIO) permits collecting information at a larger scale.
The retina is one of the most metabolically active tissues in the body: it has a continuous demand for energy supplied by ATP, the energy molecule, through glycolysis. Oxidative stress and mitochondrial dysfunction, closely linked to ATP production, may lead to retinal degeneration. Also, lipofuscin, lipids or advanced glycation end products accumulation with age seems to be accelerated in AMD, while alterations in metal homeostasis (particularly zinc and iron) have been shown to have deleterious effects in the retina. All these phenomena have been implicated in AMD development, and therefore these molecules or compounds are potential candidates to study using an approach known as “targeted” metabolomics, where the researcher looks for the presence of previously known molecules in the tissue of interest and compares it with a reference (usually, a patient with no AMD). On the other hand, an “untargeted” study represents another approach where the entire metabolic profile is analyzed to determine differences between AMD and non-AMD patients. Therefore, a priori hypotheses are not needed with the latter approach.
It is hoped that this variety of instruments and strategies to evaluate the molecular composition of tissues will eventually lead to biomarkers that can identify patients that are going to develop AMD even before the earliest vision problems and fundus changes occur. In this scenario, preventative strategies may avoid the development of this blinding disorder.
For a deeper discussion of these issues, please see: Brown CL et al. Metabolomics in age-related macular degeneration. Metabolites 2018; 9(1).
Author: Marc Biarnés OD MPH PhD, member of the BMF research team
