An open-label investigator research trial to study the safety and efficacy of a combination of a pro re nata regimen with fixed interval regimen intravitreal injections of ranibizumab in the treatment of choroidal neovascularization in subjects with neovascular age-related macular degeneration
The objective of this project is to explore the safety and efficacy of a combination of a pro re nata regimen with a fixed interval regimen of intravitreal ranibizumab injections in the treatment of choroidal neovascularization (CNV) in subjects with neovascular age-related macular degeneration. Hypothesis: This regimen may have a benefit in the range of the monthly regimen but with a significantly smaller mean number of injections.
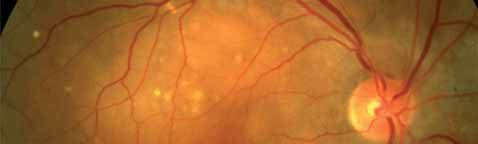
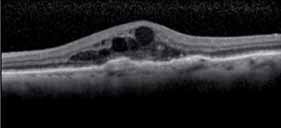
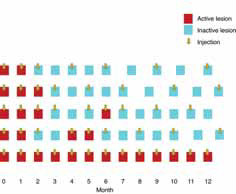
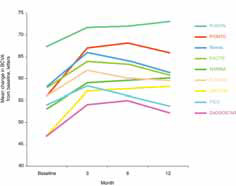
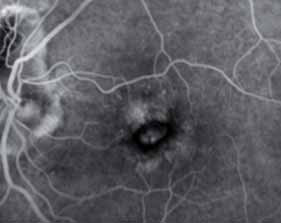
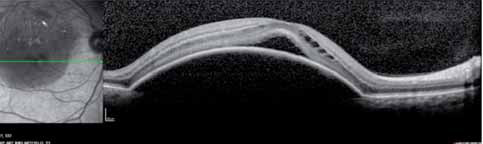
The 12-month data presented support the hypothesis that the FUSION regimen for CNV secondary to exudative AMD provides visual benefit in the range of the monthly regimens but with a significantly smaller mean number of injections, even in patients in the current incident population presenting in clinical practices with higher levels of visual acuity, not reflected in previous trials, and therefore avoiding the visual limitations of the pro re nata regimens in AMD.
Monés J, Biarnés M, Trindade F, Casaroli-Marano R. FUSION regimen: ranibizumab in treatment-naïve patients with exudative agerelated macular degeneration and high visual acuity. Graefes Arch Clin Exp Ophthalmol 2012 Dec;250(12):1737-44.
Monés J. A Review of Ranibizumab Clinical Trial Data in Exudative Age-Related Macular Degeneration and How to Translate It into Daily Practice. Ophthalmologica 2011;225(2):112-119.