MICROBEYEOME
If you are interested in taking part in a clinical trial, please send us your personal details here and we will assess whether you are eligible.
Description
Age-related macular degeneration (AMD) is the leading cause of vision loss in people of over 50 in developed countries. Although the risk factors for the disease have been widely catalogued, its causes continue to be little known.
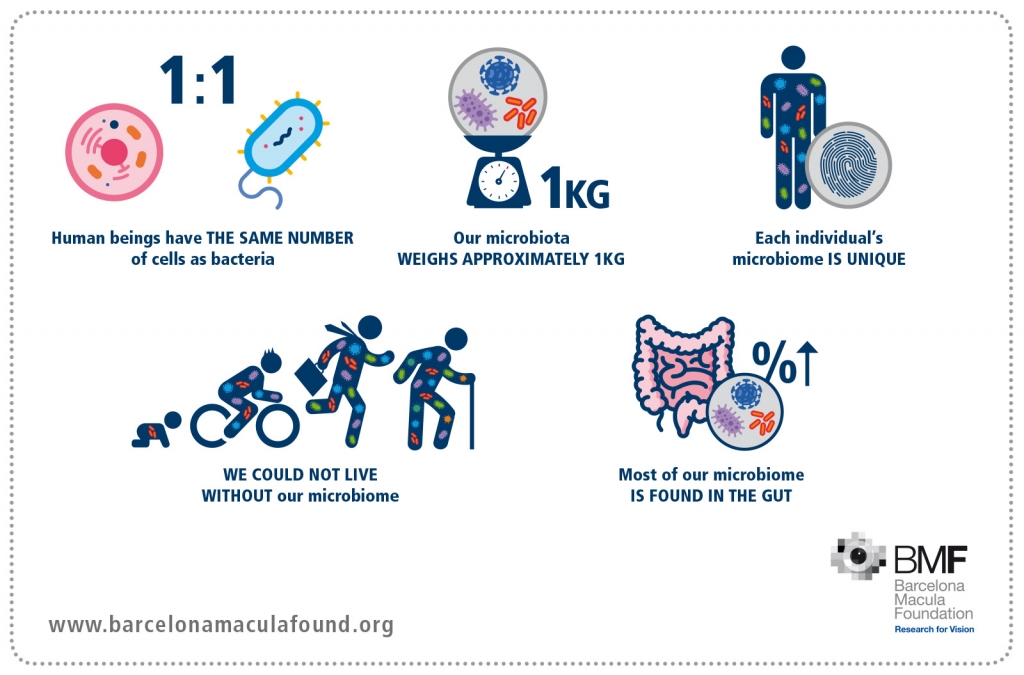
In recent years, there has been an exponential increase in research on the association between the intestinal microbiota and the diseases that affect humans.
Principal inclusion criteria
Patients of over 50, with age-related macular degeneration and healthy patients.
The patients must not have taken antibiotics in the past three months.
Patients with other neurodegenerative diseases such as Alzheimer’s disease or Parkinson’s disease, or those in treatment for any type of cancer, will be excluded.
Aim
The MICROBEYEOME study attempts to characterise the intestinal and oral microbiome of the patients diagnosed with AMD through RNA 16S sequencing and compare it with the microbiome of healthy patients, thereby identifying whether a single microbial composition may be associated with a specific phenotype. Given that the intestinal microbiome is potentially modifiable, this study could open new avenues of research for the prevention and/or possible treatment of AMD.
Duration
This initial phase of the trial consists of a single visit.