Phase IV study to evaluate the efficacy of a vegf trap-eye in subjects with neovascular age-related macular degeneration (wamd), without optimal response to repeated monthly intravitreal injections of anti vegf-a therapy
Despite the success of anti-VEGF A drugs, some patients do not respond optimally even at monthly doses with little gain or even loss of visual acuity, as well as persistent or increasing fluid in the retina.

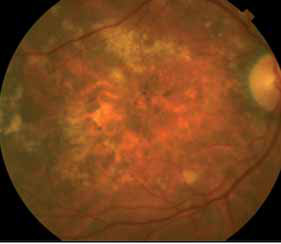
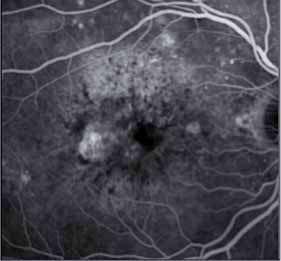
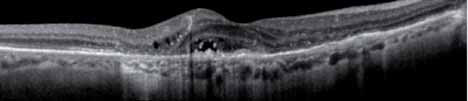
Aflibercept (VEGF Trap-Eye), a VEGF receptor fusion protein, has been recently approved by the FDA for wAMD. VEGF Trap-Eye has a higher binding affinity and a different mode of action which not only blocks VEGF-A like the current treatment, but also blocks PIGF and VEGF-B, thus blocking both VEGFR-1 and VEGFR-2 angiogenic effects. VEGF Trap-Eye given every 8 weeks after 3 monthly loading doses, has been shown to be non inferior in terms of VA results to monthly ranibizu-mab during the first year of treatment, but it offers a much lower cli-nical burden for patients, caregivers and physicians. Due to its different mode of action, its longer duration of activity and its higher affinity for ligands, VEGF Trap-Eye offers a a good opportunity to explore an alternative therapy to investigate in those patients who do not fully respond to current anti VEGF therapy.