An open-label investigator research grant trial to establish the safety of intravitreous injections of e10030 (anti-pdgf pegylated aptamer) given in combination with lucentis® in subjects with neovascular age-related macular degeneration
The objectives of this study are to evaluate the safety and efficacy of E10030 intravitreous injection when administered in combination with Lucentis® against a control of Lucentis® alone in subjects with subfoveal choroidal neovascularization secondary to age-related macular degeneration (AMD) in poor-responders to anti-VEGF monotherapy.


Open label, non-controlled
E10030 (0.3 mg) + Lucentis® for 3 months
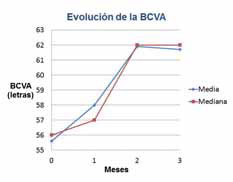
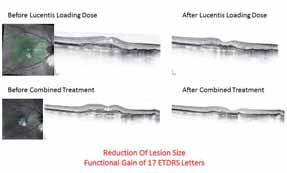
The results were that patients who were previously poor-responders to Lucentis® monotherapy, after receiving the combination of Fovista (1.5 mg) and Lucentis®, gained a mean of 6.1 letters of vision on the ETDRS standardized chart at 12 weeks. Some patients showed marked lesion reduction. No significant safety issues were observed. Data under analysis pending publication.