A phase I study to establish the safety and tolerability of arc1905 (anti-c5 aptamer) in subjects with dry age-related macular degeneration
The objectives of this study are to evaluate the safety and tolerability of ARC1905 intravitreous injections in subjects with geographic atrophy secondary to dry age-related macular degeneration (AMD). Patients were assigned in an alternating fashion to one of two dose levels of ARC1905: 0.3 or 1 mg/eye. Patients were treated with 3 initial intra-vitreal injections of ARC1905 at Day 0, Week 4, Week 8, and a follow-up visit at Week 16. They then received 2 subsequent injections at Week 24 and Week 36, with a final follow-up visit at Week 48. There was a maximum of 5 ARC1905 injections.
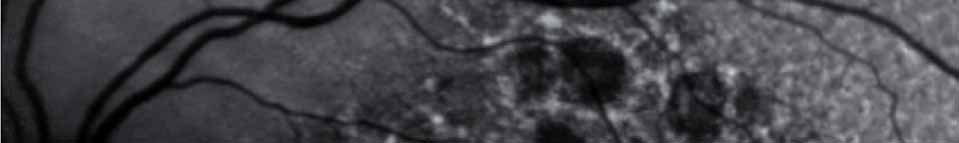
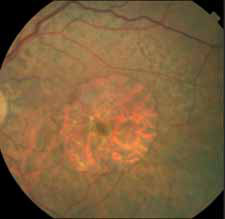
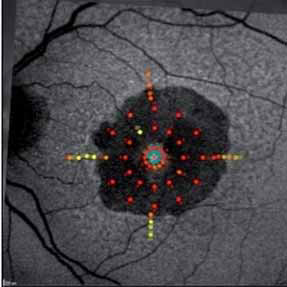
Pending analysis data. No safety concerns in this phase I intravitreal use for the first time of aptamer anti C5 for geographic atrophy.