A phase II, randomized, double-masked, controlled trial to establish the safety and efficacy of intravitreous injections of e10030 (anti-pdgf pegylated aptamer) given in combination with lucentis® in subjects with neovascular age-related macular degeneration
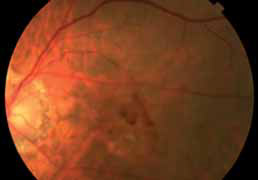
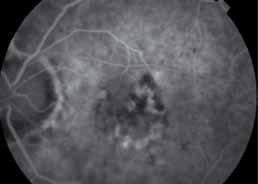
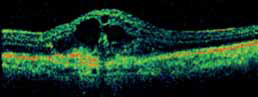
The objectives of this study are to evaluate the safety and efficacy of E10030 intravitreous injection when administered in combination with Lucentis® against a control of Lucentis® alone in subjects with subfoveal choroidal neovascularization secondary to age-related macular degeneration (AMD).
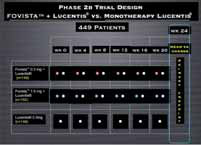


Subjects randomized in a 1:1:1 ratio to the following dose groups:
• E10030 0.3 mg/eye + Lucentis® 0. 5 mg/eye
• E10030 1.5 mg/eye + Lucentis® 0. 5 mg/eye
• E10030 sham + Lucentis® 0. 5 mg/eye
Subjects treated with active E10030 or sham E10030 in combination with Lucentis® at Day 0, Week 4, Week 8, Week 12, Week 16 and Week 20. All subjects had a final follow-up visit at Week 24.
Patients receiving the combination of Fovista (1.5 mg) and Lucentis® gained a mean of 10.6 letters of vision on the ETDRS standardized chart at 24 weeks, compared to 6.5 letters for patients receiving Lucentis® monotherapy (p=0.019), representing a 62% additional benefit. No significant safety issues were observed for either treatment group in the trial.