The crystal study: a 24-month, phase iiib, open-label, single arm, multicenter study assessing the efficacy and safety of an individualized, stabilization criteria-driven pro re nata dosing regimen with 0.5-mg ranibizumab intravitreal injections applied as monotherapy in patients with visual impairment due to macular edema secondary to central retinal vein occlusion (crvo)
This study will provide additional efficacy and safety data for 0.5-mg ranibizumab using pro re nata dosing over 24 months in patients with visual impairment due to macular edema secondary to CRVO.

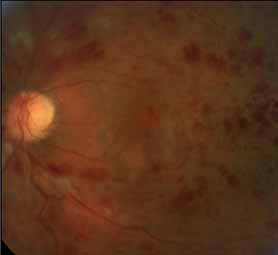
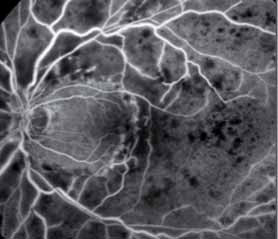
Spectral domain high-definition optical coherence tomography (OCT) images will be analyzed to gain insights into predictive factors for disease progression and the possibility of reduced monitoring will be assessed in Year 2. The results of this open-label study will provide long-term safety and efficacy data to further guide recommendations on the use of ranibizumab in this indication. The primary objective is to evaluate the efficacy of an individualized stabilization criteria-driven PRN dosing regimen with 0.5 mg ranibizumab as assessed by the mean best-corrected visual acuity (BCVA) change at Month 12 compared to Baseline.