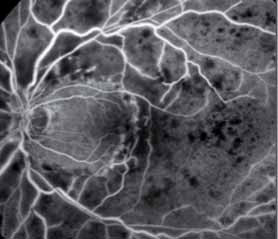
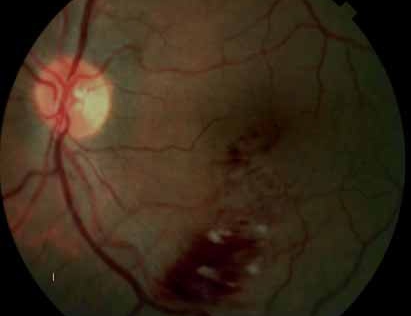
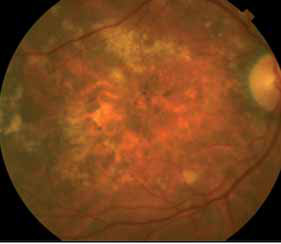
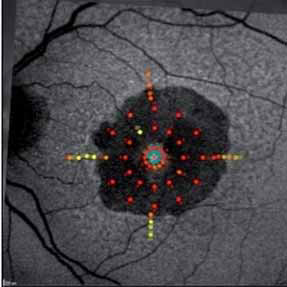
The crystal study: a 24-month, phase iiib, open-label, single arm, multicenter study assessing the efficacy and safety of an individualized, stabilization criteria-driven pro re nata dosing regimen with 0.5-mg ranibizumab intravitreal injections applied as monotherapy in patients with visual impairment due to macular edema secondary to central retinal vein occlusion (crvo)
Barcelona Macula Foundation
Institut de la Màcula i de la Retina
And 70 more clinical sites worldwide